Powder-George YL*
Department of Chemistry, The University of the West Indies, St. Augustine, Trinidad and Tobago
- Yomica L Powder-George
Department of Chemistry, The University of the West Indies
St. Augustine, Trinidad and Tobago
Tel: +18686626013
Fax: +18686453771
E-mail: pyomica@hotmail.com
Received Date: May 11, 2017; Accepted Date: May 17, 2017; Published Date: May 22, 2017
Citation: Powder-George YL (2017) Luteolin-7-O-Glycosides from the Leaves of Centropogon cornutus (Campanulaceae). Nat Prod Chem Res 5:271. doi: 10.4172/2329-6836.1000271
Copyright: © 2017 Powder-George YL. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Natural Products Chemistry & Research
View PDF Download PDF
Abstract
A new luteolin-7-O-glycoside, named Centropogin A (1), together with three known luteolin-7-O-glycosides, Luteolin- 7-O-xyloside (2), Luteolin-7-O-[β-apiofuranosyl-(1→2)]β-xylopyranoside (3) and Luteolin-7-O-[β-glucopyranosyl- (1→2)-α-rhamnosyl-(1→6)] β-glucopyranoside (4) were isolated and purified via repeated chromatography of the n-BuOH soluble fraction from the methanol extract of the leaves of Centropogon cornutus (Campanulacae). The structures of these compounds were elucidated by analysis of their 1D-NMR (1H, 13C) and 2D-NMR (COSY, HSQC, HMBC), MS (HR-ESIMS) spectral data, and by comparisons with the literature data. This is the first report of secondary metabolites from the genus Centropogon and the first report of compounds (2)-(4) from the Campanulaceae family.
Keywords
Campanulaceae; Centropogon cornutus; Flavonoids; Luteolin-7-O-glycosides; Centropogin A; NMR; HR-ESIMS
Introduction
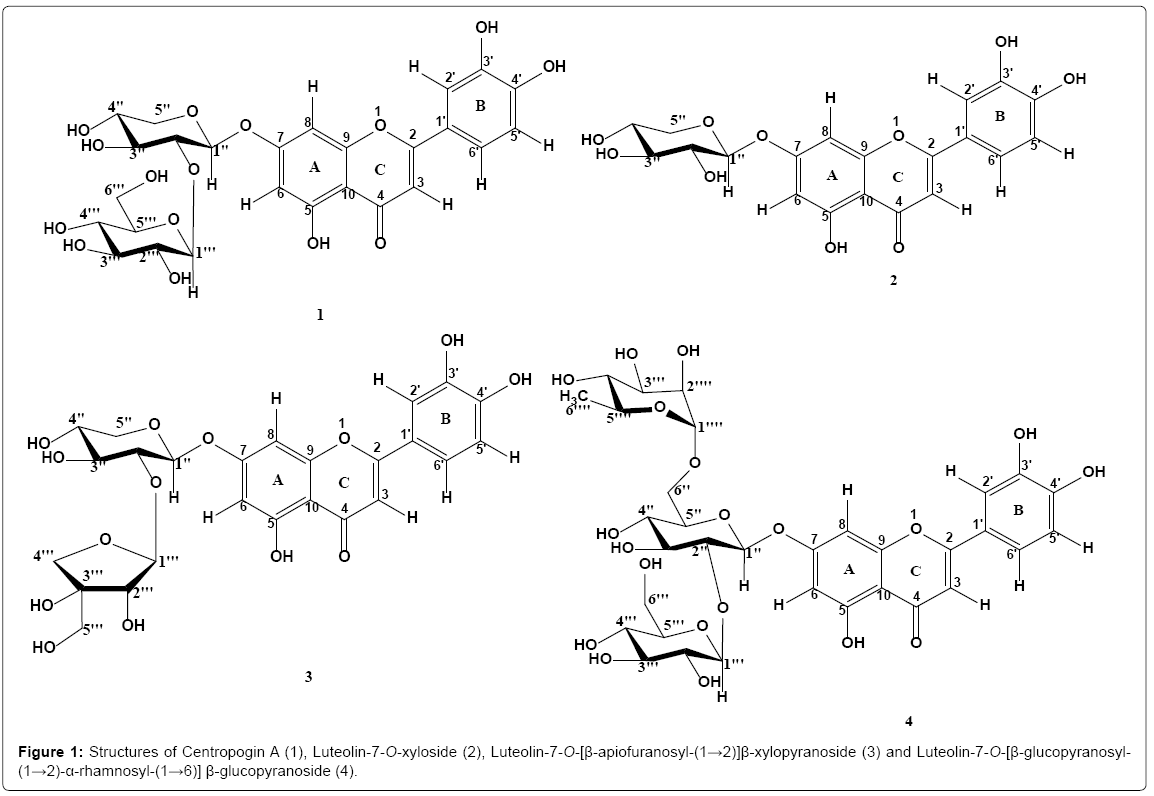
Campanulaceae, the bellflower family, is a well-known group of plants comprising 84 genera and approximately 2400 species [1]. The family is cosmopolitan in its distribution and present in a wide array of habitats [2]. A few species in the family belonging to the genera Adenophora, Codonopsis, Lobelia and Platycodon have previously been analyzed with respect to their secondary metabolites and biological activities, and a diverse range of compounds have been isolated to date. These include pyrrolidine, piperidine and polyhydroxylated alkaloids, steroidal saponins, triterpenoids, phenylpropanoids, flavonoids, xanthones, polyacetylenes and cerebrosides, some of which exhibited significant anticancer, antimicrobial and anti-inflammatory activities [3-8]. Centropogon, the third largest genus of Campanulaceae, comprises of 230 species [9]. Centropogon cornutus is the only Centropogon species found in Trinidad and Tobago, where it is commonly known as deer meat, deer bush and crepe coq [10]. These showy roadside plants, generally found along moist shady banks and cocoa fields, are distributed throughout Central and South America and the West Indies [10]. Centropogon cornutus is a shrub with a milky sap that grows to about 1-3 meters in height. It has curved scarlet flowers, which are suited for pollination by hummingbirds, and produces berries which are consumed by birds [11]. Traditionally, the leaves are used to treat snake and scorpion bites in Trinidad [12]. In Guyana, the entire plant is boiled and the liquid drank to remedy venereal diseases. In French Guiana, the leaves are used as a tonic and as an abortive decoction, while in NW Guyana it is used to treat urinary tract problems [13]. The whole plant is also traditionally used to treat stomach ulcers in Brazil [14]. Many chemical and pharmacological investigations focusing on a few genera of Campanulaceae have been carried out previously, however, there is limited information on the pharmacological or phytochemical composition of the Centropogon genus which has until now not been investigated for its natural products. As a continuation of the search for secondary metabolites of biological importance from Campanulaceae species, herein, the isolation and structural elucidation of one new compound and three known compounds from the n-BuOH soluble fraction from the methanol extract of the leaves of C. cornutus is reported. Their structures were elucidated using spectroscopic methods including NMR and HR-ESIMS as the novel Centropogin A (1), together with three known luteolin glycosides, Luteolin-7-Oxyloside (2), Luteolin-7-O-[β-apiofuranosyl-(1→2)]β-xylopyranoside (3) and Luteolin-7-O-[β-glucopyranosyl-(1→2)-α-rhamnosyl-(1→6)] β-glucopyranoside (4) which are all for the first time reported from the Campanulaceae family (Figure 1).
Experimental Section
General experimental procedures
NMR spectra were recorded on a Bruker AVANCE DRX-600 instrument with TMS as internal standard. HRESIMS was carried out on a micrOTOF-Q (Bruker Daltonics) spectrometer. Column chromatography (CC) was performed on Silica gel 60 Å 70-230 mesh (Sigma-Aldrich) and VLC was performed using Silica gel 60 PF254+366 (Merck). Analytical TLC was carried out on aluminium-backed plates precoated with 0.20 mm thick silica gel (Sigma-Aldrich) and visualization of the chromoplates was facilitated by UV light. The solvents used for extraction and chromatographic separations were either of analytical grade or distilled prior to use.
Plant material
The leaves of Centropogon cornutus investigated in this study were collected on the Guaico Tamana road, Trinidad in August 2013. Identification and botanical authentication was performed by Ms. Keisha Manuare of the National Herbarium of Trinidad and Tobago and a voucher specimen (TRIN 50133) was deposited.
Extraction and isolation
The leaves of Centropogon cornutus were oven-dried for 3 days at 40°C (473 g), pulverized and extracted exhaustively with MeOH. The methanolic solution was filtered and concentrated in vacuo to yield 159 g of a viscous, dark-brown extract which was suspended in MeOH/H2O (4:1) and partitioned sequentially with Petroleum ether, CHCl3, EtOAc and n-BuOH, respectively. The n-BuOH extract (22 g) was subjected to vacuum liquid chromatography (VLC) on silica gel (Silica gel 60 PF254+366) and eluted with EtOAc: MeOH: H2O gradients of increasing polarity (95:3:2, 90:6:4, 80:12:8) to give 9 fractions (1A– 1I). Fraction 1B, a pure compound was assigned as compound 2 (22.1 mg). Fraction 1E (0.9227 g) was purified by isocratic silica gel column chromatography using the system EtOAc: MeOH: H2O (90:6:4) to yield compound 3 (155.9 mg). 1G (0.5025 g) was subjected to isocratic silica column chromatography using the system EtOAc: MeOH: H2O (90:6:4) to afford compound 1 (12.4 mg) and Fraction 1I (10.124 g) was chromatographed on a gradient elution silica column chromatography using the mobile phase CHCl3: MeOH (75:25, 65:35) to give compound 4 (52.9 mg).
Centropogin A (1): Yellow amorphous powder, yielded a positive response to the Shinoda test; HRESIMS (positive mode) m/z 603.1310 [M+Na]+ (calcd. for C26H28O15Na, 603.1320); 1H-NMR (DMSO-d6, 600 MHz) and 13C NMR (DMSO-d6, 150 MHz) data (Table 1).
| Position | δC | δH, multiplicity |
|---|---|---|
| Aglycone | ||
| 2 | 164.3 | ------- |
| 3 | 103.2 | 6.74, s |
| 4 | 181.8 | ------- |
| 5 | 161.3 | ------- |
| 6 | 99.3 | 6.46, d (2.0) |
| 7 | 162.5 | ------- |
| 8 | 94.4 | 6.82, d (2.0) |
| 9 | 156.8 | ------- |
| 10 | 105.3 | ------- |
| 1ʹ | 121.2 | ------- |
| 2ʹ | 113.7 | 7.42, d (2.0) |
| 3ʹ | 145.7 | ------- |
| 4ʹ | 150.1 | ------- |
| 5ʹ | 116 | 6.89, d (8.0) |
| 6ʹ | 119.1 | 7.46, dd (8.0, 2.0) |
| 5-OH | ------- | 12.99, bs |
| 7-O-Xylose | ||
| 1ʺ | 98.3 | 5.25, d (6.8) |
| 2ʺ | 81.8 | 3.53, m |
| 3ʺ | 74.8 | 3.50, m |
| 4ʺ | 68.6 | 3.47, m |
| 5ʺ | 65.2 | 3.80, d (6.7) 3.45, m |
| 2ʺ-O-Glucose | ||
| 1‴ | 104.4 | 4.46, d (7.9) |
| 2‴ | 74.5 | 3.00, t (8.4) |
| 3‴ | 76.1 | 3.20, m |
| 4‴ | 69.4 | 3.14, m |
| 5‴ | 76.6 | 3.17, m |
| 6‴ | 60.2 | 3.43 (CH2) |
aData acquired at 600 MHz (1H) and 150 MHz (13C) in DMSO-d6 with TMS as the internal standard.
Table 1: NMR Spectral data for Compound 1 (DMSO-d6, δ in ppm, J in Hz).a
Luteolin-7-O-xyloside (2): Yellow amorphous powder, yielded a positive response to the Shinoda test; HRESIMS (positive mode) m/z 441.1863 [M+Na]+ (calcd. for C20H18O10Na, 441.0792); 1H-NMR (DMSO-d6, 600 MHz) δ: 12.99 (1H, bs, OH-5), 7.46 (1H, dd, J=8.5, 2.0, H-6′), 7.42 (1H, d, J=2.0, H-2′), 6.88 (1H, d, J=8.5 Hz, H-5′), 6.78 (1H, d, J=2.0, H-8), 6.74 (1H, s, H-3), 6.41 (1H, d, J=2.0, H-6), 5.08 (1H, d, J=7.0 Hz, Xyl-H-1), 3.26 (1H, d, J=6.3 Hz, Xyl-H-2), 3.77 (1H, d, J=6.0 Hz, Xyl-H-4); 13C-NMR (DMSO-d6, 150 MHz) δ: 164.5 (C-2), 103.0 (C-3), 181.9 (C-4), 161.1 (C-5), 100.2 (C-6), 162.7 (C-7), 94.5 (C-8), 157.0 (C-9), 105.4 (C-10), 121.1 (C-1′), 113.4 (C-2′), 145.9 (C-3′), 149.8 (C-4′), 116.0 (C-5′), 119.3 (C-6′), 99.5 (Xyl-1), 72.9 (Xyl-2), 76.2 (Xyl- 3), 69.2 (Xyl-4), 65.8 (Xyl-5).
Luteolin-7-O-[β-apiofuranosyl-(1→2)] β-xylopyranoside (3): Yellow amorphous powder, yielded a positive response to the Shinoda test; HRESIMS (negative mode) m/z 549.1093 [M-H]+ (calcd. for C25H25O14, 549.1239); 1H-NMR (DMSO-d6, 600 MHz) δ: 12.99 (1H, bs, OH-5), 7.46 (1H, dd, J=8.0, 2.0, H-6′), 7.44 (1H, d, J=2.0, H-2′), 6.91 (1H, d, J=8.0 Hz, H-5′), 6.76 (1H, d, J=2.0, H-8), 6.75 (1H, s, H-3), 6.39 (1H, d, J=2.0, H-6), 5.19 (1H, d, J=7.6 Hz, Xyl-H-1), 5.34 (1H, bs, Api-H-1); 13C-NMR (DMSO-d6, 150 MHz) δ: 164.5 (C-2), 103.1 (C- 3), 181.9 (C-4), 161.2 (C-5), 99.3 (C-6), 162.4 (C-7), 94.5 (C-8), 156.9 (C-9), 105.4 (C-10), 121.3 (C-1′), 113.6 (C-2′), 145.8 (C-3′), 150.0 (C- 4′), 116.0 (C-5′), 119.2 (C-6′), 98.5 (Xyl-1), 75.7 (Xyl-2), 76.6 (Xyl-3), 69.4 (Xyl-4), 65.7 (Xyl-5), 108.9 (Api-1), 76.1 (Api-2), 79.3 (Api-3), 74.0 (Api-4), 64.2 (Api-5).
Luteolin-7-O-[β-glucopyranosyl-(1→2)-α-rhamnosyl-(1→6)] β-glucopyranoside (4): Yellow amorphous powder, yielded a positive response to the Shinoda test; αD25 =-0.60 (c 0.1, MeOH); HRESIMS (positive mode) m/z 779.1914 [M+Na]+ (calcd. for C33H40O20Na, 779.2005); 1H-NMR (DMSO-d6, 600 MHz) δ: 13.00 (1H, bs, OH-5), 7.42 (1H, dd, J=8.0, 2.0, H-6′), 7.37 (1H, d, J=2.0, H-2′), 6.86 (1H, d, J=8.0 Hz, H-5′), 6.79 (1H, d, J=2.0, H-8), 6.71 (1H, s, H-3), 6.48 (1H, d, J=2.0, H-6), 5.22 (1H, d, J=7.0 Hz, Glc-H-1), 4.48 (1H, d, J=7.5 Hz, Glc′-H-1), 4.55 (1H, bs, Rha-H-1), 1.07 (3H, d, J=6.1 Hz, Rha-H-6); 13C-NMR (DMSO-d6, 150 MHz) δ: 164.5 (C-2), 103.4 (C-3), 181.8 (C- 4), 161.1 (C-5), 99.6 (C-6), 162.6 (C-7), 95.1 (C-8), 156.8 (C-9), 105.4 (C-10), 121.2 (C-1′), 113.9 (C-2′), 146.0 (C-3′), 151.4 (C-4′), 115.3 (C- 5′), 119.2 (C-6′), 98.4 (Glc-1), 82.5 (Glc-2), 75.5 (Glc-3), 69.2 (Glc-4), 75.3 (Glc-5), 65.8 (Glc-6), 104.7 (Glc′-1), 74.7 (Glc′-2), 76.2 (Glc′-3), 69.6 (Glc′-4), 76.9 (Glc′-5), 60.6 (Glc′-6), 100.5 (Rha-1), 70.3 (Rha-2), 70.8 (Rha-3), 72.1 (Rha-4), 68.3 (Rha-5), 17.8 (Rha-6).
Results and Discussion
Purification of the n-BuOH soluble fraction of the crude MeOH extract of the leaves afforded one new compound, Centropogin A (1) and three known compounds (2-4). The three known compounds were identified as Luteolin-7-O-xyloside (2) [15,16], Luteolin-7-O- [β-apiofuranosyl-(1→2)]β-xylopyranoside (3) [17], and Luteolin-7-O- [β-glucopyranosyl-(1→2)-α-rhamnosyl-(1→6)] β-glucopyranoside (4) [17], by comparison of their spectral data with those published in the literature.
Compound 1 was obtained as an amorphous, yellow powder which gave a positive response to Shinoda test [18]. The HR-ESIMS exhibited a pseudo-molecular ion peak at m/z 603.1310 [M+Na]+ (calcd. for C26H28O15Na, 603.1320) corresponding to the molecular formula of C26H28O15, which implies 13 degrees of unsaturation.
The 1H-NMR spectrum of compound 1 exhibited six aromatic proton signals (Table 1). A one-proton meta-coupled doublet at δ 7.42 ppm (J=2.0 Hz), a one-proton ortho-coupled doublet at δ 6.89 ppm (J=8.0 Hz) and a one-proton ortho-, meta-coupled doublet of doublets at δ 7.46 ppm (J=2.0, 8.0 Hz) were ascribed to aromatic protons H-2′, H-5′ and H-6′, respectively, supporting an ABX-type coupling system of ring B. It also showed two meta–coupled doublets (J=2.0 Hz) at δ 6.82 and 6.46, each integrating for one proton, which were assigned to H-8 and H-6, respectively of ring A of 5,7-dihydroxyflavonoids. A one-proton sharp singlet at δ 6.74 ppm was ascribed to H-3. These spectral data revealed the presence of a 5, 7, 3', 4' tetrahydroxy Flavone aglycone also known as Luteolin. The presence of a downfield chelated hydroxy proton signal at δ 12.99 (1H, bs) confirmed the presence of a hydroxy group at position 5 of ring-A. Two anomeric proton signals were also observed at δ 5.25 (1H, d, J=6.8 Hz) and 4.46 (1H, d, J=7.9 Hz), suggesting the presence of two sugar moieties. According to the coupling constants of H-1′′ (J=6.8 Hz) and H-1′′′ (J=7.9 Hz), the sugar moieties were assigned as β-anomers.
The 13C NMR spectrum of 1 also exhibited signals due to the flavone aglycone and two sugar moieties. The 13C NMR data showed the presence of a ketone carbonyl (δ 181.8), two olefinic carbons (δ 164.3 and 103.2), four hydroxyl carbons (δ 162.5, 161.3, 150.1 and 145.7) and two anomeric carbons (δ 104.4 and 98.3).
A combined interpretation of the 1H-1H COSY, 13C NMR, HSQC and HMBC spectra and of the coupling constants aided the identification of the sugars as one β-glucose and one β–xylose moiety (Table 1).
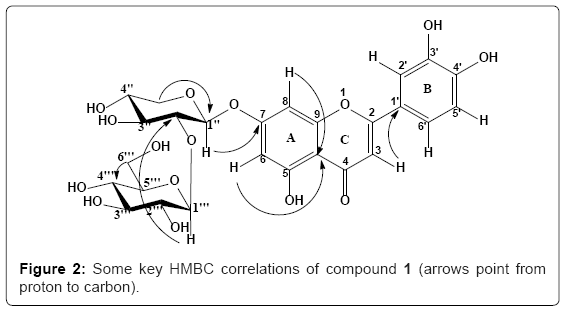
The sequence of the sugars and binding site at the aglycone were unambiguously determined in the HMBC experiment. In the HMBC spectrum, significant correlations were observed between H-1′′ (δ 5.25) and C-7 (δ 162.5); H-1′′′ (δ 4.46) and C-2′′ (δ 81.8), suggesting the xylose moiety was located at C-7 position of the aglycone, and the glucosyl was connected with C-2′′ of the xylosyl, thus establishing the inter-glycosidic linkage of the disaccharide moiety (Figure 2). Thus, compound 1 was identified as Luteolin-7-O-[β-glucopyranosyl-(1→2)] β-xylopyranoside, and was named Centropogin A.
Conclusion
From the leaves of Centropogon cornutus, one novel flavone glycoside (1) is reported as well as three flavone glycosides (2-4) are described for the first time in the Campanulaceae family. Luteolin-7- O-xyloside (2) is reported to exhibit antimutagenic activity [16]. This work contributes to the knowledge of the chemistry of the Centropogon genus which is non-existent to date. It would be interesting to examine if these flavonoids are responsible for the traditional medicinal uses of this plant.
Acknowledgements
The author wishes to thank the Department of Chemistry, The University of the West Indies, St. Augustine, Trinidad for providing the necessary facilities to carry out this work and Ms. Keisha Manuare of the National Herbarium of Trinidad and Tobago for plant identification. The author also acknowledges Mrs. Tahirah Sanderson and Ms. Whitney Raghoo for their technical assistance.
Disclosure Statement
The author has declared no conflict of interest.
References
- Schneeweiss GM, Pachschwöll C, Tribsch A, Schönswetter P, Barfuss MH, et al. (2013) Molecular phylogenetic analyses identify Alpine differentiation and dysploid chromosome number changes as major forces for the evolution of the European endemic Phyteuma (Campanulaceae). Molecular Phylogenetics and Evolution 69: 634-652.
- Antonelli A (2008) Higher level phylogeny and evolutionary trends in Campanulaceae subfam. Lobelioideae: Molecular signal overshadows morphology. Molecular Phylogenetics and Evolution 46: 1-18.
- Asano N, Nishida M, Miyauchi M, Ikeda K, Yamamoto M, et al. (2000) Polyhydroxylated pyrrolidine and piperidine alkaloids from Adenophora triphylla var. japonica (Campanulaceae). Phytochemistry 53: 379-382.
- He JY, Ma N, Zhu S, Komatsu K, Li ZY, et al. (2015) The genus Codonopsis (Campanulaceae): a review of phytochemistry, bioactivity and quality control. Journal of Natural Medicines 69: 1-21.
- Dar AA, Dangroo NA, Raina A, Qayum A, Singh S, et al. (2016) Biologically active xanthones from Codonopsis ovata. Phytochemistry 132: 102-108.
- Zhao B, Ren J, Yuan Z (2013) Isolation of a new cerebroside from Codonopsis lanceolata. Biochemical Systematics and Ecology 46: 26-28.
- Kim YS, Kim JS, Choi SU, Kim JS, Lee HS, et al. (2005) Isolation of a new saponin and cytotoxic effect of saponins from the root of Platycodon grandiflorum on human tumor cell lines. Planta medica 71: 566-568.
- Ma Y, Wink M (2008) Lobeline, a piperidine alkaloid from Lobelia can reverse P-gp dependent multidrug resistance in tumor cells. Phytomedicine 15: 754-758.
- Harvey Y (1992) Centropogon cornutus: Campanulaceae. Curtis's Botanical Magazine 9: 3-7.
- Winer L (2009) Dictionary of the English/Creole of Trinidad & Tobago: On Historical Principles. McGillQueen's Press, MQUP.
- Kenny J (2006) Flowers of Trinidad and Tobago. Prospect Press.
- Lans C (2007) Comparison of plants used for skin and stomach problems in Trinidad and Tobago with Asian ethnomedicine. J Ethnobiol Ethnomed 3: 3.
- DeFilipps RA, Maina SL, Crepin J (2004) Medicinal plants of the Guianas (Guyana, Surinam, French Guiana). Washington, DC: Department of Botany, National Museum of Natural History, Smithsonian Institution.
- Voeks RA (1997) Sacred leaves of Candomblé: African magic, medicine, and religion in Brazil. University of Texas Press.
- Lin YL, Wang CN, Shiao YJ, Liu TY, Wang WY (2003) Benzolignanoid and polyphenols from Origanum vulgare. Journal of the Chinese Chemical Society 50: 1079-1083.
- Gulluce M, Karadayi M, Guvenalp Z, Ozbek H, Arasoglu T, et al. (2012) Isolation of some active compounds from Origanum vulgare L. ssp. vulgare and determination of their genotoxic potentials. Food Chemistry 130: 248-253.
- Koffi EN, Le Guernevé C, Lozano PR, Meudec E, Adjé FA, et al. (2013) Polyphenol extraction and characterization of Justicia secunda Vahl leaves for traditional medicinal uses. Industrial Crops and Products 49: 682-689.
- Shinoda J (1928) A new biologically active flavone glycoside from the roots of Cassia fistula Linn. J Pharm Soc Jpn 48:214.